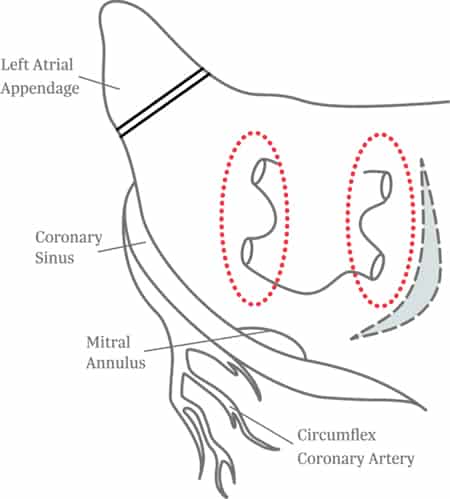
Lesion Set Options
To treat Atrial Fibrillation using ablative technologies, there are
four lesion sets that surgeons typically use.
See Lesion Set Options
During the Ablate AF Trial, 84% of patients were free of AFib symptoms at 6 months1. Complications are reported to be less than 1% for surgical ablation2. Complications may include bleeding, infection, blood clots, kidney failure, and cardiac arrhythmia3.
Maze Procedure
Heart
Surgical
33257
Class 1
The Maze procedure ablates the heart using radiofrequency or cryo energy to restore the heart to normal sinus rhythm
Atrial Fibrillation
ABLATE AF
AtriCure, Inc.
NCT01694563
http://schema.org/Cardiovascular
Approved
The primary objective of this post-approval study is to evaluate the clinical outcomes in a cohort of patients with non-paroxysmal forms of atrial fibrillation (persistent or long-standing persistent) treated during commercial use of the AtriCure Synergy Ablation System by physicians performed the Maze IV procedure.
References
- Philpott, J.M. et al. (2015). The ABLATE Trial: Safety and Efficacy of Cox Maze-IV Using a Bipolar Radiofrequency Ablation System. Ann of Thorac Surg, 100(5):1541-8.
https://www.ncbi.nlm.nih.gov/pubmed/26387721 - StopAfib. Maze Procedure Risks [Internet]. StopAfib.org. 2009.
https://www.stopafib.org/maze-risks.cfm - Medicine JH. Atrial Fibrillation Surgery [Internet]. Johns Hopkins Medicine. https://www.hopkinsmedicine.org/healthlibrary/test_procedures/cardiovascular/atrial_fibrillation_surgery_135,313
Page last updated: June 10, 2019